Fundada en el 1995
De Barcelona para el mundo
En más de 80 países
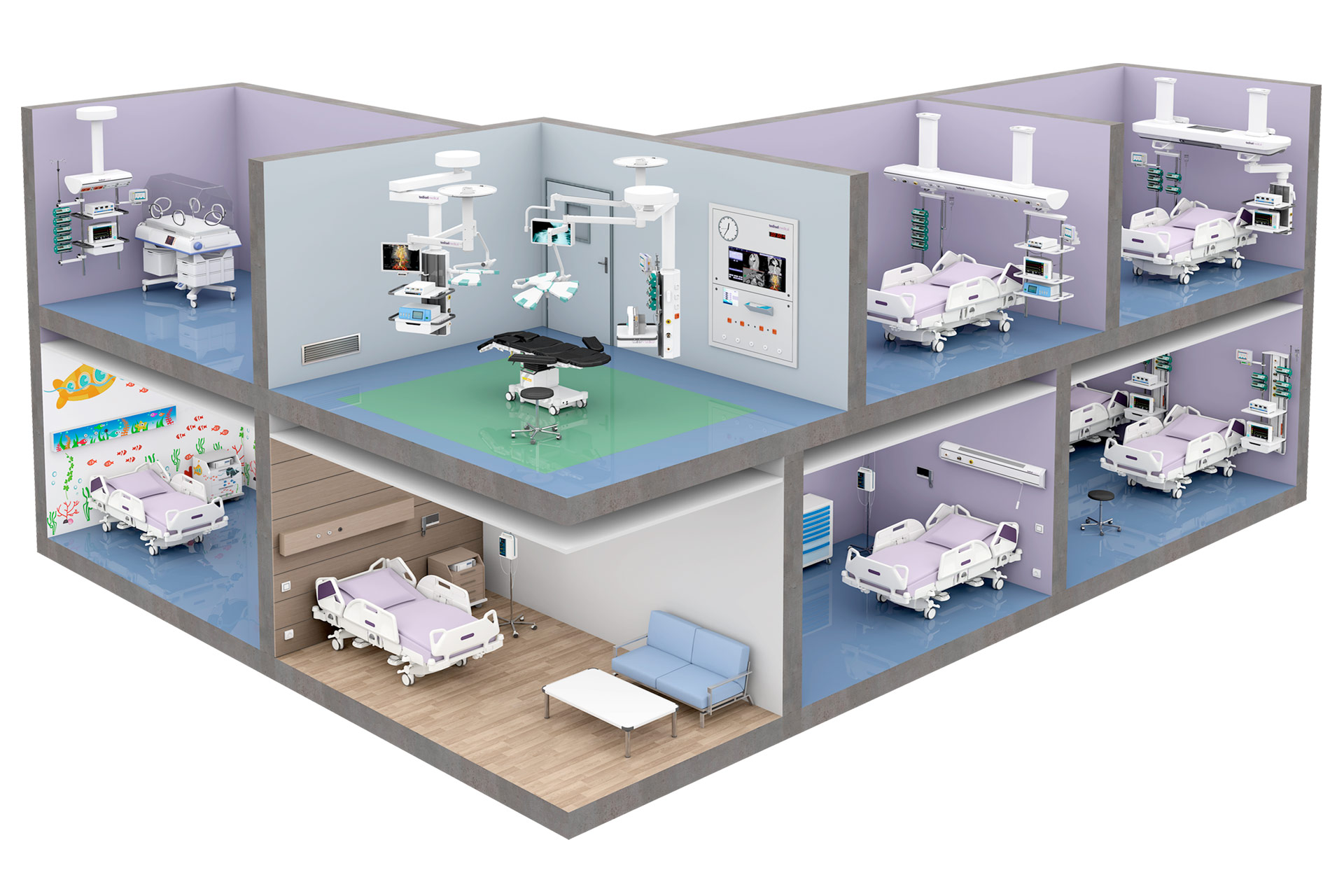
Fabricamos equipamiento hospitalario para zonas de hospitalización, áreas críticas y bloque quirúrgico.
Tedisel es internacionalmente conocida por su gran variedad y adaptabilidad de productos, especialmente por la fabricación de Cabeceros, Suspendidos para UCI, Columnas, Paneles y Software para quirófanos.
4 pasos de nuestra historia de éxito
- ESCUCHAMOS Y ASESORAMOS01Gracias a la estrecha relación con nuestros clientes, nos centramos en detectar los nuevos retos de cada mercado y encontrar juntos la mejor solución.
- DISEÑAMOS02Nuestros expertos diseñadores e ingenieros dedican sus esfuerzos para crear las soluciones más económicas y eficientes para cada área, hospital y país.
- FABRICAMOS03Nuestra experiencia en la fabricación desde 1995, el talentoso personal y las eficientes máquinas, llevan las ideas imaginadas al mundo real.
- ESCUCHAMOS DE NUEVO04Después de entregar la solución, volvemos a escuchar. Aparte de las nuevas necesidades detectadas, ¡la mejora e innovación de las soluciones actuales no debe detenerse nunca!
| AMÉRICA | |
| Bolivia Chile Colombia Costa Rica Cuba Ecuador | Nicaragua Panamá Perú Rep. Dominicana Trinidad y Tobago |
| ÁFRICA | |
| Argelia Angola Camerún Egipto Gabón Ghana Guinea Ecuatorial Libia Madagascar Mauritania | Marruecos Nigeria Rep. Centroafricana Rep. Democrática Congo Sierra Leona Sudán Túnez |
| EUROPA | |
| Alemania Andorra Bélgica Bulgaria Chipre Croacia República Checa Dinamarca España Estonia Finlandia Francia Grecia Irlanda | Italia Letonia Lituania Macedonia Malta Noruega Países Bajos Polonia Portugal Reino Unido Rumanía Rusia Suiza Ucrania |
| ORIENTE MEDIO | |
| Arabia Saudí Bahréin Irak Israel Jordania Kuwait | Líbano Omán Palestina Qatar Emiratos Árabes Unidos |
| ASIA | |
| Bangladesh Brunéi China Filipinas Hong Kong | India Indonesia Pakistán Sri Lanka Vietnam |
Calidad e innovación en equipamiento hospitalario
Explíquenos su proyecto y un equipo experto le asesorará con la solución idónea
Al día del sector hospitalario









Celebramos nuestro más reciente proyecto en colaboración con el valioso grupo Quirónsalud
7 tendencias de desarrollo energético hospitalario en 2024
Innovación en Acceso a la Atención Médica con Tedisel Medical Software